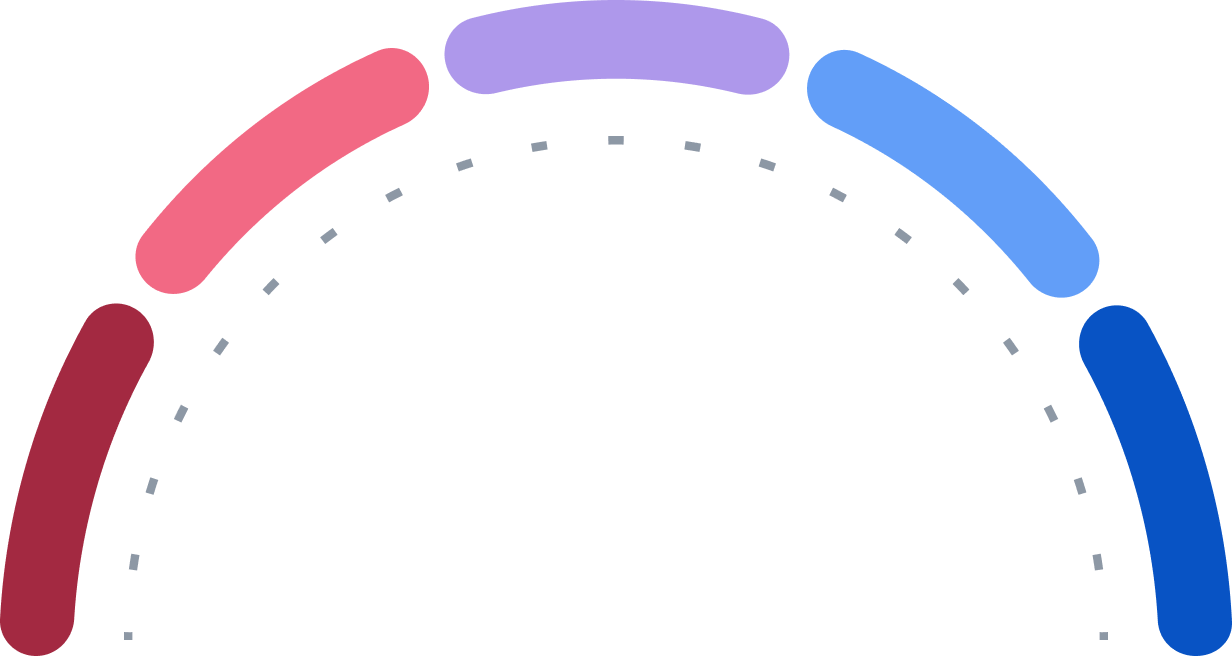
Buy
Strong Buy
Buy
Hold
Sell
Strong Sell
0
14
5
0
0


| Exchange | Ticker | Currency | Last Trade | Price | Daily Change |
|---|---|---|---|---|---|
 NASDAQ |
VSTM
|
USD
|
28.01.2026 14:50
|
6,34 USD
| -0,16 USD
-2,46 %
|
 London |
0LOV.L
|
USD
|
28.01.2026 14:30
|
6,45 USD
| -0,05 USD
-0,74 %
|
 Frankfurt |
2VSA.F
|
EUR
|
28.01.2026 14:25
|
5,25 EUR
| 0,15 EUR
+2,94 %
|
 Hamburg |
VIRSN35.HAMB
|
EUR
|
28.01.2026 07:12
|
5,40 EUR
| 0,30 EUR
+5,88 %
|
 Quotrix |
VIRSN35.DUSD
|
EUR
|
28.01.2026 06:27
|
5,45 EUR
| 0,35 EUR
+6,86 %
|
 Düsseldorf |
VIRSN35.DUSB
|
EUR
|
27.01.2026 18:30
|
5,15 EUR
| -0,05 EUR
-0,96 %
|
 Frankfurt
Frankfurt
| Name | Symbol |
|---|---|
| Düsseldorf | VIRSN35.DUSB |
| Frankfurt | 2VSA.F |
| Hamburg | VIRSN35.HAMB |
| London | 0LOV.L |
| NASDAQ | VSTM |
| Quotrix | VIRSN35.DUSD |