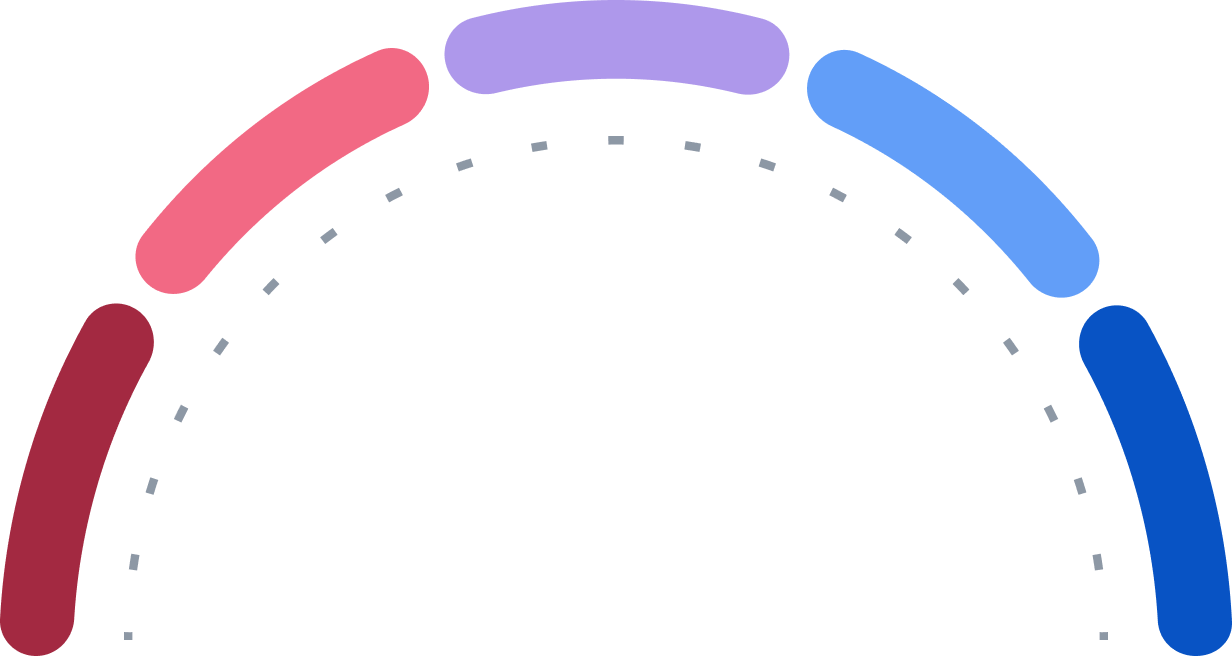
Hold
Strong Buy
Buy
Hold
Sell
Strong Sell
0
9
14
1
0


The following funds have invested in IVERIC BIO INC:
Fund | Vol. in million 35,23 | Percentage (%) 0,08 % |
 NASDAQ
NASDAQ
| Date | From | To |
|---|---|---|
| 17.04.2019 | OPHT | ISEE |
| Name | Symbol |
|---|---|
| NASDAQ | ISEE |