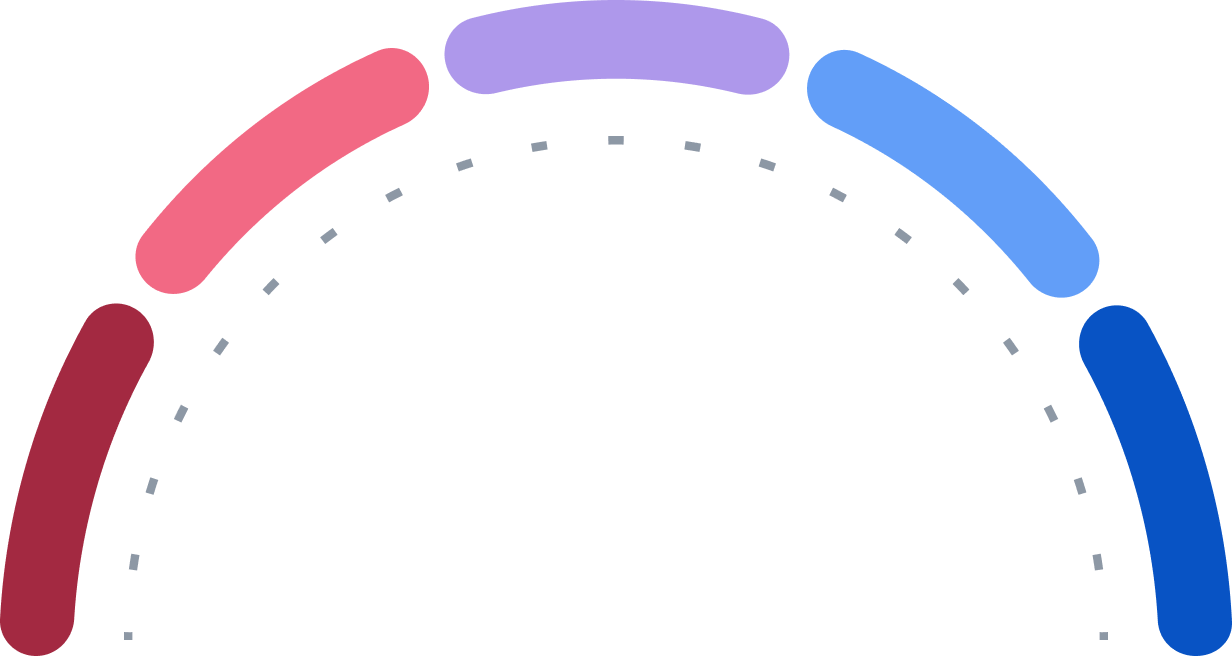
Achat
Achat fort
Achat
Conserver
Vente
Vente forte
1
24
1
1
0


| Bourse | Ticker | Devise | Dernier échange | Cours | Variation journalière |
|---|---|---|---|---|---|
 NASDAQ |
XNCR
|
USD
|
04.02.2026 17:03
|
11,75 USD
| -0,24 USD
-2,02 %
|
 Frankfurt |
XE9.F
|
EUR
|
04.02.2026 07:04
|
9,70 EUR
| -0,15 EUR
-1,52 %
|
 Quotrix |
XIRSDL57.DUSD
|
EUR
|
04.02.2026 06:27
|
10,10 EUR
| 0,25 EUR
+2,54 %
|
 Düsseldorf |
XIRSDL57.DUSB
|
EUR
|
03.02.2026 18:30
|
9,70 EUR
| 0,10 EUR
+1,04 %
|
Les fonds suivants ont investi dans XENCO INC :
Fonds | Vol. en millions 64,85 | Part (%) 0,15 % |
 NASDAQ/NMS (GLOBAL MARKET)
NASDAQ/NMS (GLOBAL MARKET)
| Nom | Symbole |
|---|---|
| Düsseldorf | XIRSDL57.DUSB |
| Frankfurt | XE9.F |
| NASDAQ | XNCR |
| Quotrix | XIRSDL57.DUSD |