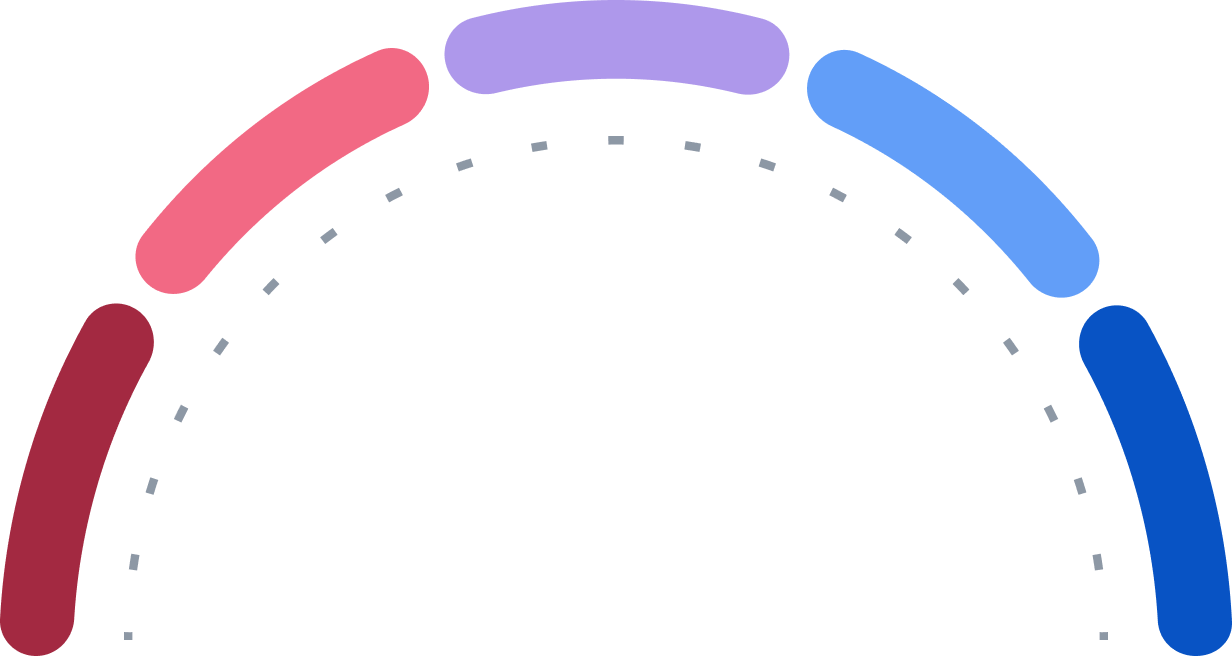
Buy
Strong Buy
Buy
Hold
Sell
Strong Sell
0
14
5
0
0


| Exchange | Ticker | Currency | Last Trade | Price | Daily Change |
|---|---|---|---|---|---|
 NASDAQ |
VSTM
|
USD
|
19.12.2025 23:00
|
7,99 USD
| 0,24 USD
+3,08 %
|
 Frankfurt |
2VSA.F
|
EUR
|
19.12.2025 14:29
|
6,45 EUR
| -0,05 EUR
-0,77 %
|
 Quotrix |
VIRSN35.DUSD
|
EUR
|
19.12.2025 06:27
|
6,60 EUR
| 0,10 EUR
+1,54 %
|
 Düsseldorf |
VIRSN35.DUSB
|
EUR
|
18.12.2025 18:30
|
6,65 EUR
| -0,45 EUR
-6,34 %
|
 Hamburg |
VIRSN35.HAMB
|
EUR
|
18.12.2025 07:08
|
6,75 EUR
| -0,35 EUR
-4,93 %
|
 London |
0LOV.L
|
USD
|
08.12.2025 17:24
|
10,08 USD
| -0,28 USD
-2,68 %
|
 Frankfurt
Frankfurt
| Name | Symbol |
|---|---|
| Düsseldorf | VIRSN35.DUSB |
| Frankfurt | 2VSA.F |
| Hamburg | VIRSN35.HAMB |
| London | 0LOV.L |
| NASDAQ | VSTM |
| Quotrix | VIRSN35.DUSD |